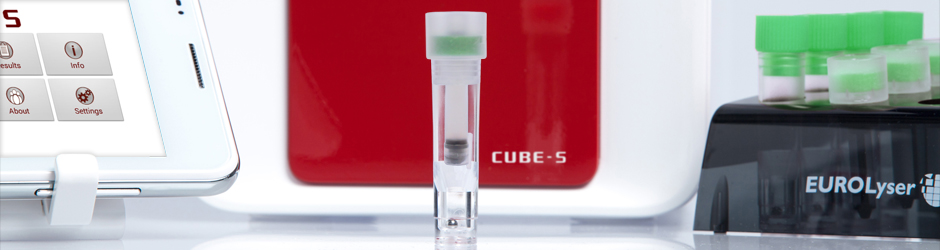
The Eurolyser smart PT (INR) test system is an easy-to-use, flexible point-of-care laboratory photometer that is capable of monitoring patients that are treated under permanent anticoagulation therapy. The rapid tests provide exact results that are comparable to advanced laboratory standards – which are documented in the attached evaluation.
The Eurolyser smart PT (INR) test system is an easy-to-use, flexible point-of-care laboratory photometer that is capable of monitoring patients that are treated under permanent anticoagulation therapy. The rapid tests provide exact results that are comparable to advanced laboratory standards – which are documented in the attached evaluation.
The compact, easy-to-use instrument and its tests provide a comfortable way of measurement of blood coagulation time respectively the determination of PT (INR) parameter from only 20µl capillary blood. Routine measurements of the PT (INR) parameter ensure a secure and effective adjustment of the e.g. Phenprocoumon-(Marcumar®) dose anticoagulated patients receive.
Product Advantages
- Easy-to-use and easily readable due to big touch screen
- PT (INR) determination in few minutes with only 20µl capillary blood from finger tip
- Increased patient satisfaction through blood collection from finger tip
- Maximizes efficiency in doctor’s office: no more re-scheduling of result discussions with patients
- Connectable to LIS or HIS software
- Operator ID and/or Patient ID input with bar code reader possible
- Flexible point-of-care instrument: Single instrument is capable of processing 11 different tests e.g. CRP, Ferritin, µAlbumin, D-Dimer, …
- more than 5.000 GPs and laboratories worldwide use the Eurolyser smart instrument
| Contact us for further information | Watch our product video |
3 easy steps to the PT (INR) result:
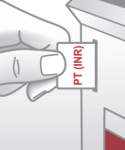 Insert RFID card into |
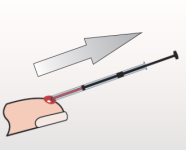 Collect sample and dispense into cuvette. |
 Place cuvette into analyser – |
Technical data
- Test results comparable to laboratory standards
- Assay range: 1,0 – 6,0 INR
- Precision within run: < 5%
- Test duration: 2:30 minutes at INR 1
- Shelf life of tests up to 12 months
- 250 patient data storage
- RS 232 and USB interface – connection for PC and/or LIS
- weight: 3,4 kg
- size: 26 x 14,5 x 14 cm